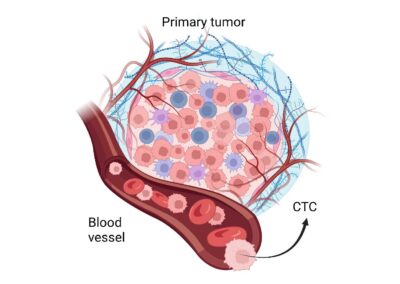
Circulating Tumor Cells (CTCs) are cancer cells that have detached from a primary tumor or have mutated into cancerous cells and are found circulating in the bloodstream. Detecting CTCs can help assess cancer risk, monitor disease progression, evaluate metastasis (the spread of cancer), and gauge how well a patient is responding to treatment.
Today, researchers have developed various techniques to detect CTCs through blood samples, including physical property-based separation, immunoaffinity (antibody binding), or a combination of both. These often involve positive and negative enrichment methods to isolate CTCs. Beyond simply isolating and counting CTCs, further analysis of these cells provides invaluable insights into cancer monitoring, treatment effectiveness, and the development of personalized or integrative oncology approaches, which aim for high efficacy, minimal side effects, and improved quality of life for cancer patients.
Oncotrace RGCC: A Practical and Insightful CTC Test
One of the most useful and non-invasive methods for CTC detection is the Oncotrace RGCC test. This test can help identify the origin of the CTCs (i.e., the primary tumor), allowing results to be used for personalized health recommendations, treatment planning, and future disease prognosis.
The process involves drawing a blood sample for lab analysis, which identifies the presence or absence of CTCs, quantifies their levels, and characterizes their immunophenotype—i.e., the types and surface markers expressed by these CTCs. This provides physicians with insight into the nature and origin of the tumor, disease progression, and overall prognosis.

Molecular and Cellular-Level CTC Analysis
Beyond the basic isolation, enumeration, and phenotyping, laboratories can conduct deeper analyses at the molecular and cellular levels of CTCs. This includes:
- Epigenetic profiling (gene expression analysis)
- Viability assays: Testing how CTCs respond to chemotherapy drugs or natural compounds by measuring their sensitivity or resistance.
These results are instrumental in designing tailored cancer treatment plans and help physicians select the most appropriate therapeutic strategies with the highest potential for a positive patient response.
Who Should Consider CTC Testing?
The risk of cancer is closer to us than we think. Understanding the body’s internal state early allows for easier prevention and disease control. CTC testing is suitable not only for current cancer patients but also as a screening tool for:
- Health-conscious individuals with high-risk lifestyles or health concerns
- People with a family history of cancer
- Those seeking proactive and preventive care even in the absence of symptoms
In summary, CTC testing provides a non-invasive, highly informative window into the presence and behavior of cancer cells in the bloodstream. It empowers both patients and physicians with timely information that can lead to early intervention, better outcomes, and truly personalized care.
- Vidlarova M, Rehulkova A, Stejskal P, Prokopova A, Slavik H, Hajduch M, Srovnal J. Recent Advances in Methods
for Circulating Tumor Cell Detection. Int J Mol Sci. 2023 Feb 15;24(4):3902. doi: 10.3390/ijms24043902. PMID:
36835311; PMCID: PMC9959336. - Deng Z, Wu S, Wang Y, Shi D. Circulating tumor cell isolation for cancer diagnosis and prognosis. EBioMedicine.
2022 Sep;83:104237. doi: 10.1016/j.ebiom.2022.104237. Epub 2022 Aug 27. PMID: 36041264; PMCID: PMC9440384. - Castro-Giner F, Aceto N. Tracking cancer progression: from circulating tumor cells to metastasis. Genome Med.
2020 Mar 19;12(1):31. doi: 10.1186/s13073-020-00728-3. PMID: 32192534; PMCID: PMC7082968. - Lin D, Shen L, Luo M, Zhang K, Li J, Yang Q, Zhu F, Zhou D, Zheng S, Chen Y, Zhou J. Circulating tumor cells:
biology and clinical significance. Signal Transduct Target Ther. 2021 Nov 22;6(1):404. doi:
10.1038/s41392-021-00817-8. PMID: 34803167; PMCID: PMC8606574. - Bhagwat N, Carpenter EL. Flow Cytometric Methods for Circulating Tumor Cell Isolation and Molecular Analysis.
Adv Exp Med Biol. 2017;994:105-118. doi: 10.1007/978-3-319-55947-6_5. PMID: 28560670. - Papasotiriou I, et al. Detection of Circulating Tumor Cells in Patients with Breast, Prostate, Pancreatic, Colon
and Melanoma Cancer: A Blinded Comparative Study Using Healthy Donors. Journal of Cancer Therapy.
2015;6:543-553. http://dx.doi.org/10.4236/jct.2015.67059 . - Guadagni S, Clementi M, Masedu F, Fiorentini G, Sarti D, Deraco M, Kusamura S, Papasotiriou I, Apostolou P,
Aigner KR, Zavattieri G, Farina AR, Vizzielli G, Scambia G, Mackay AR. A Pilot Study of the Predictive Potential
of Chemosensitivity and Gene Expression Assays Using Circulating Tumour Cells from Patients with Recurrent
Ovarian Cancer. Int J Mol Sci. 2020 Jul 7;21(13):4813. doi: 10.3390/ijms21134813. PMID: 32646060; PMCID:
PMC7370156. - Guadagni S, Masedu F, Fiorentini G, Sarti D, Fiorentini C, Guadagni V, Apostolou P, Papasotiriou I, Parsonidis
P, Valenti M, Ricevuto E, Bruera G, Farina AR, Mackay AR, Clementi M. Circulating tumour cell gene expression
and chemosensitivity analyses: predictive accuracy for response to multidisciplinary treatment of patients with
unresectable refractory recurrent rectal cancer or unresectable refractory colorectal cancer liver metastases.
BMC Cancer. 2022 Jun 16;22(1):660. doi: 10.1186/s12885-022-09770-3. PMID: 35710393; PMCID: PMC9202660.